Maximum safety when processing highly potent Antibody Drug Conjugates (ADCs) with proven filling solutions.
Antibody-Drug Conjugates represent a new frontier in oncology treatment and demand exceptional process control, containment and compliance. As your partner in aseptic manufacturing, we provide the precision, safety and reliability required to process high-potency substances under Annex 1 and FDA guidelines. Let’s find the right setup for your ADC application. Get in contact and partner with us now.Targeted therapies and their requirements for pharmaceutical production
Antibody-drug conjugates (ADCs) combine the advantages of highly potent cytostatic therapy with the selective use of targeted therapy, making them one of the most promising therapeutic approaches in oncology. The processing of highly effective ADCs requires specialized containment systems that ensure both the protection of operating personnel and product integrity. Isolator technology and suitable filtration systems play a central role.
An optimal interaction of innovative technologies, validated processes and the highest safety standards guarantee a secure and efficient filling of ADCs. As your partner in pharmaceutical production, we reliably guarantee the quality and effectiveness of the products as well as maximum product safety and operator safety.

// At Bausch+Ströbel, our mission is to make life-saving medicines available worldwide while ensuring their safe and secure production. We take this responsibility seriously by delivering intelligent technology, outstanding quality, and consistent safety for both operators and products. By combining time-tested concepts with cutting-edge innovations, we deliver solutions that offer intuitive handling, thorough cleaning of containers and machinery, and full compliance with Annex 1. However, our commitment goes further: setting new benchmarks and defining the standards of tomorrow, together with our customers. ///
Meeting the challenges of ADC manufacturing – safely and precisely
The following key areas highlight the core requirements for efficient, safe and compliant ADC manufacturing – and how our technologies are designed to meet them.
Highest operator safety / security
Minimized product loss

Hygienic design for best / easy cleanability
Exterior cleaning of the objects

GMP-/ FDA-/ Annex 1-compliant machine design
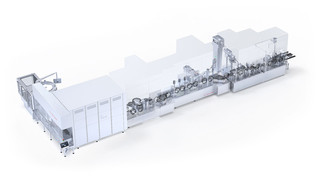
Benefit from our expertise and all-in-one solutions
Manufacturers of Antibody-Drug Conjugates (ADCs) face complex challenges: Whether you fill small batches or run full-scale production – every volume must be spot on. Strict containment and safety standards are essential to protect product quality and your team. Our machine solutions are built to meet exactly these demands – with precision, safety, and reliability.Our Business Development expert Katrin Meinikheim, is happy to discuss current trends in ADC production and show how our technologies help you to master the complexity of highly potent drug manufacturing. Let’s find the right setup for your ADC application. Get in contact and partner with us now.
 // Our FVF5063 is purpose-built for the demanding requirements of ADC fill-finish.
// Our FVF5063 is purpose-built for the demanding requirements of ADC fill-finish.
It ensures aseptic safety and product integrity through in-line vial inspection, AdvancedFill technology to reduce product loss, and vacuum-free stopper insertion—including re-stoppering. Its washdown-capable, hygienic design supports highly potent APIs with full GMP, FDA, and Annex 1 compliance. All critical process steps are protected by unidirectional First Air in accordance with Annex 1, ensuring sterile conditions at the point of highest risk. A dedicated pressure zone concept further safeguards both operators and product—making the FVF5063 a trusted solution for advanced aseptic processing. ///
Why choose high potent FVF5063 for ADCs?
- Inspection of the vials during feeding to avoid any risk of contamination in the isolator area
- Minimization of product loss through AdvancedFill technology
- Availability of various dosing systems
- Aseptic set-up of the bowl-free-feed (BFF) stopper sorting with closed, biodecontaminated isolator
- Vacuum-free machine concept
- Vacuum-free stopper insertion station incl. re-stopping function
- Hygienic design through-transport using “diamond kinematics” for better cleaning
- Washdown-capable machine concept with cleaning recommendation for highly potent active ingredients
- External cleaning of objects
- Minimization of critical areas through zone separation and pressure zone concepts
- Pressure zone concepts protect operators and ensure aseptic processing conditions
- Dedicated pressure zone between sorting and filling/stoppering reduces exposure risk
- Complete GMP, FDA and Annex 1 conformity
