// Maximum safety in HPAPI and ADC processing with proven filling solutions. ///
What are HPAPIs?
HPAPIs stands for ‘Highly Potent Active Pharmaceutical Ingredients’. These substances are highly biologically active, which means that even the smallest amounts can have a therapeutic effect.
Key aspects of HPAPIs:
- High potency: Often used in oncology, e.g. in cancer drugs.
- Low therapeutic dose: Even microgram quantities can be effective.
- Strict safety requirements: Manufacturing and handling require special protective measures (e.g. closed systems, isolators, special protective clothing).
- Toxicity: HPAPIs are dangerous to people if inhaled or absorbed through the skin.
Examples
ADCs – Precision in oncology
Antibody-Drug Conjugates (ADCs) are a subgroup of HPAPIs and are among the most innovative classes of active ingredients in cancer treatment. They combine the targeted binding of an antibody with the power of a highly potent active ingredient in the form of a cytostatic agent or toxin. The result: precise delivery of active ingredients directly to tumor cells and their destruction, with the added benefit that healthy cells are spared as much as possible, since the goal is to eliminate only the tumor cells.ATMPs – The future of personalized medicine
Advanced Therapy Medicinal Products (ATMPs) include, among other things, cell and gene therapies. Some of these therapeutics are highly individualized and offer new therapeutic approaches for diseases that were previously difficult or impossible to treat.In addition to HPAPIs, there are a range of highly selective tools and technologies for the treatment of cancer, autoimmune diseases, and genetic disorders. These advanced options also support tissue repair, such as regenerating damaged cartilage or restoring heart tissue affected by a heart attack.
ATMPs, including cell and gene therapies, may qualify as HPAPI due to their high potency and specific handling requirements. Common features include small batch sizes and elevated safety risks from gene vectors, viral carriers, and allergenic potential—especially upon mucosal contact. Strict containment and protective measures are essential.
Challenges in processing highly potent active ingredients
The manufacture and processing of highly potent active pharmaceutical ingredients (HPAPIs) involves special requirements for pharmaceutical companies.Among the greatest challenges are:
- Occupational safety and containment
HPAPIs are effective even in very small doses – maximum protective measures for employees and the environment are therefore essential. Without adequate containment, there is a risk of exposure that could endanger not only health but also regulatory approval.
- Technological requirements
Conventional open production facilities are not sufficient for handling HPAPIs. To ensure both safety and product quality, closed systems and precise dosing technologies are absolutely essential for secure processing.
- Regulatory complexity
The global requirements of the FDA, EMA and other authorities demand complete documentation, valid processes and the consistent implementation of GMP standards at the highest level.
- Product stability and quality assurance
Maintaining the stability of highly potent and sensitive substances plays a crucial role, particularly in ADCs and ATMPs. Due to their biological nature, they are more susceptible to external influences – even the smallest deviations in product purity, temperature or process control can impair effectiveness.
Solutions from Bausch+Ströbel for processing

// At Bausch+Ströbel, our mission is to make life-saving medicines available worldwide while ensuring their safe and secure production. We take this responsibility seriously by delivering intelligent technology, outstanding quality, and consistent safety for both operators and products. By combining time-tested concepts with cutting-edge innovations, we deliver solutions that offer intuitive handling, thorough cleaning of containers and machinery, and full compliance with Annex 1. However, our commitment goes further: setting new benchmarks and defining the standards of tomorrow, together with our customers. ///
Markus Ströbel, Member of the Executive Board | Bausch+Ströbel
Meeting the challenges of HPAPI manufacturing – safely and precisely
The following key areas highlight the core requirements for efficient, safe and compliant HPAPI manufacturing – and how our technologies are designed to meet them.
Highest operator safety / security
Minimized product loss

Hygienic design for best / easy cleanability
Exterior cleaning of the objects

GMP-/ FDA-/ Annex 1-compliant machine design
GENEX - Aseptic small-batch production reimagined
Whether ADCs or ATMPs: new forms of therapy demand sterility with minimum batch sizes and high product value. Our fully automatic GENEX filling and packaging system is specifically designed for this purpose.The system operates entirely without gloves and without manual intervention. Robots perform all critical steps: from format changes and biomonitoring to troubleshooting. This minimizes human error, which is considered the most common source of non-compliance and a significant contamination risk, and ensures consistently high product quality.
GENEX combines advanced cleanroom robotics with a modular design. This enables the development of adaptable processes for customized Fill & Finish applications, ranging from clinical trials to the production of small batches for commercial use.

The benefits:
- Ensuring maximum product and patient safety by complying with strict sterility requirements in accordance with Annex 1
- The risk of particle and germ contamination is minimized due to robotic gloveless isolator operation. Furthermore, potential leaks, damage or incorrect operation at the glove ports are eliminated.
- Robot-assisted, GMP-compliant format changes offer maximum flexibility
- Suitable for clinical trials and small batches of various product types and volumes.
- Accelerated approval procedures due to reproducible and validatable processes
GENEX sets new standards in the aseptic filling of complex biologics, especially ATMPs and ADCs with cytotoxic payloads.
Safe. Precise. Highly potent.
The solution for the commercial filling of HPAPIs.
The commercial production of HPAPIs such as ADCs or ATMPs requires more than sterility. It demands speed, safety, flexibility and maximum productivity. The FVF 5063 filling and closing machine is designed to meet these requirements.With up to 24,000 vials per hour, 100% in-process control and minimal product loss, it is ideal for filling high-value biologics. The proven AdvancedFill technology saves valuable active ingredients during start-up and shutdown – for maximum cost-effectiveness.
With its modular dosing systems, using peristaltic pumps or a time-pressure system, the FVF 5063 can be flexibly adapted to different requirements. A well-designed diamond-shaped transport system ensures gentle handling of even sensitive containers as well as hygienic design.
The FVF 5063 also delivers impressive performance in terms of aseptic processing: our aseptic stopper feed system does not require a sorting bowl and, due to its sophisticated design, enables optimum first-air immersion – in full compliance with Annex 1. FVF 5063 represents scalable performance, GMP compliance, and process reliability in the commercial production of ADCs.
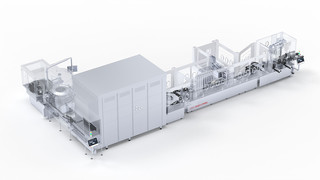
Why choose FVF5063 for HPAPIs?
- Vial Inspection to avoid any risk of contamination in the isolator area
- Zero reject principle with AdvancedFill technology and vacuum-free re-stoppering function
- Aseptic set-up of the bowl-free-feed (BFF) stopper sorting with closed, bio-decontamination isolator
- Hygienic design and washdown-capable machine concept
- External cleaning of objects to thoroughly remove product residues from their outer surfaces
- Advanced pressure zone concepts protecting operators and ensure aseptic processing conditions
- Complete GMP, FDA and Annex 1 conformity
