// GENEX: The gloveless generation for aseptic processing. ///
The production of viral vectors is expensive and technically demanding – especially in the Fill & Finish sector. It is therefore extremely important that no waste is produced during this process step and that sources of error are eliminated. Around 50% of all deviations are caused by human error. Automated, glove-free aseptic systems are therefore indispensable. They reduce the risk of errors, increase throughput and ensure the availability of therapies.
Our new fully automated GENEX filling and packaging system offers the perfect solution for precisely these challenges. Our team of Engineers and Process Experts has thus developed a novel approach that significantly reduces the risk of contamination, thereby defining new safety standards in aseptic processing.
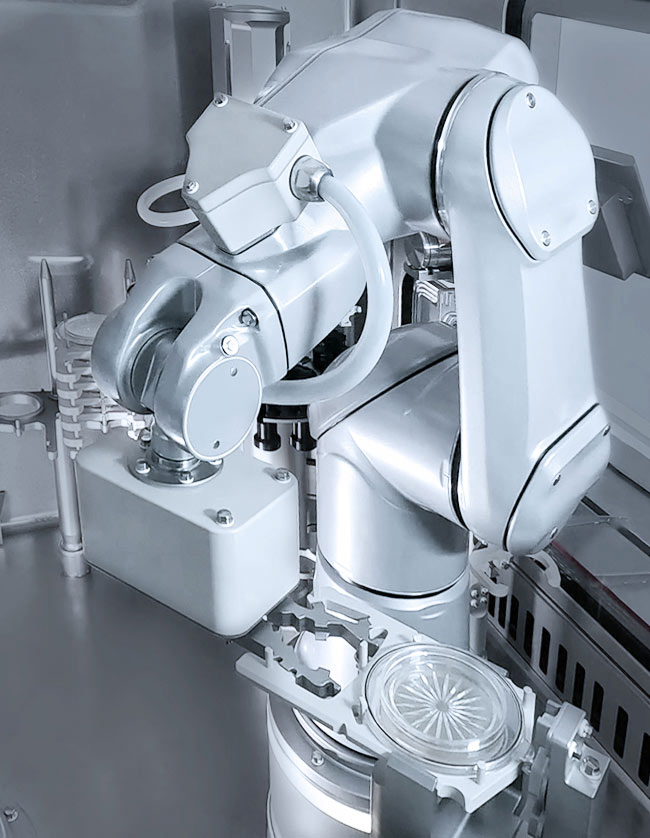
To meet the challenge of processing ATMPs, GENEX works completely gloveless and without manual intervention – by using robot technology. This not only ensures maximum sterility, but also maximum reliability and reproducibility.
GENEX is the result of state-of-the-art, fully integrated automation. Precision automation has been proven to deliver better and safer results than relying on human intervention. GENEX thus enables efficient production while ensuring maximum process and production reliability.

// The pharmaceutical industry is now facing a new challenge:
it must be able to flexibly process increasingly smaller batches. ///
Markus Ströbel, Member of the Executive Board | Bausch+Ströbel

Scaleable

Next level hygienic design

Eliminates human interaction
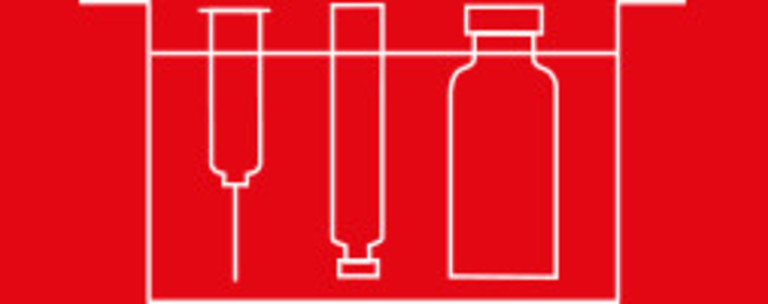
Processes various packaging materials

GMP, FDA, Annex 1 conform

High process reliability
Minimized product loss

Space & time efficient
100% robotic – automation at every critical step
Until now, the use of cleanroom robots has been largely limited to the handling, preparation and transport of packaging materials. There are far greater potential applications: From bio-monitoring of the production environment to fully automated format changes and troubleshooting. These and other process steps are carried out with GENEX using robot technology.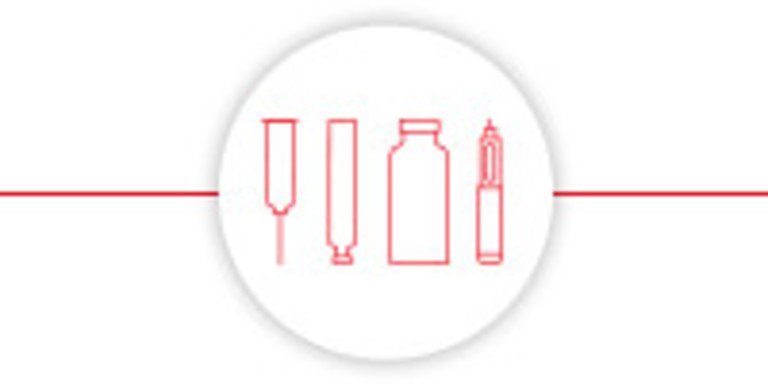
Container
handling
Filling &
stoppering
Environmental
monitoring
Surface
monitoring
Format
changes


What our customers say
Christa Myers
CRB
“Bausch+Ströbel is one of our trusted partners, and one of the reasons we keep coming back to Bausch+Ströbel is their ability to deliver exactly what the customer needs in terms of flexibility.”
“Bausch+Ströbel is one of our trusted partners, and one of the reasons we keep coming back to Bausch+Ströbel is their ability to deliver exactly what the customer needs in terms of flexibility.”
Benefit from our expertise: Better production processes for new therapies
For small batch sizes, fast, safe, and of course compliant changes to the production setup are crucial. GENEX has a modular design and can be flexibly combined to form individual process chains – for tailor-made solutions for new forms of therapy:- ATMPs: Cell and gene therapy, stem cell products, personalised medicine
- Orphan Drugs: Treatment of rare diseases with high product value of therapy units
- Clinical Trial Supply: clinical trial drugs for all phases of studies
- Commercial small-batch production: across all drug groups
Annex 1 Conformity and GENEX
The FDA recommends the increased use of robotics to increase the level of automation and reduce both the number and complexity of human interventions. The new Annex 1 offers technological latitude, but makes it clear that robots can significantly reduce interventions in the Class A zone – or, as in the case of GENEX, even avoid them altogether. This not only protects operators from highly potent substances, but also significantly increases process reliability and product quality through reproducible, validatable processes. The cleanability of the systems is also subject to new requirements. The pharmaceutical compliance team has therefore completely revised GENEX's hygienic design and consistently aligned it with current regulations.Maximum safety in HPAPI and ADC processing with proven filling solutions


